Background:
Hodgkin's Lymphoma (HL), especially classic (cHL) subtype has a favorable prognosis and frontline therapy in almost 70-80% reach a durable complete remission. However, 10-30 % of patients relapse after achieving response, and an additional almost 14-15% will never achieve complete remission and will have refractory disease. Both groups need a second line chemotherapy regimen and possible transplantation. Among 2 nd line chemotherapy regimens, a combination of ifosfamide (I), carboplatin (C), and etoposide (E) is preferred. The aim of this systematic review is to study the efficacy and safety of combinations of ICE with other drugs in relapsed/refractory (RR) HL patients.
Methods:
We followed PRISMA guidelines to conduct this systematic review. A literature search was performed on PubMed, Embase, and Clinicaltrials.gov with mesh terms, “Hodgkin Lymphoma” and “ICE” from the inception of data till 07/06/2023. We screened 1412 articles and included 2 phase II randomized clinical trials (N=43) and 4 nonrandomized clinical trials (N=109), measuring the efficacy and safety of combinations of ICE in RR HL. Data was extracted for overall response rate (ORR), complete response (CR), partial response (PR), overall survival (OS) and progression free survival (PFS).
Results:
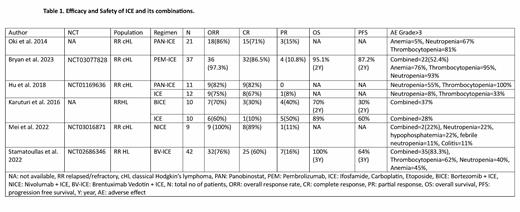
In the included clinical trials (N=152), ICE was combined with Immune check point inhibitors (ICIs) in two clinical trials on Pembrolizumab (PEM) and Nivolumab (N) (N=46). Panobinostat (PAN) was combined with ICE in phase I and phase I/II trials (N=32). Bortezomib (B) (N=10) and Brentuximab Vedotin (BV) (N=42) were studied in randomized phase II and phase I in combination with ICE, respectively. In 2 clinical trials (N=46) on combinations of ICE with ICIs, ORR, CR, and PR was 97-100%, 86-89%, and 11%. Grade≥3 hematological toxicity was 52% and 22% in PEM-ICE and NICE, respectively. The 2-year PFS and OS were 72% (95% CI: 56-83) and 95% (95% CI: 82-99), respectively in NICE study. BV-ICE showed ORR, CR, and PR of 76%, 60% and 16%, respectively with a 3-year OS and PFS of 100% and 64%, respectively. Combined grade ≥3 adverse events were 83.3%. PAN-ICE showed ORR, CR, and PR of 82-86%, 71-82%, 15% in phase I and II clinical trials but at the cost of greater myelosuppression depicted by thrombocytopenia's in 80-100% of patients. On the other hand, ICE alone showed ORR, CR, and PR of 75%, 67%, and 8%, respectively without causing significant myelosuppression. BICE showed ORR, CR, and PR of 70%, 30%, and 40%, respectively in comparison to ICE ORR, CR, and PR of 60%, 10% and 50%, respectively. BICE group showed the OS and PFS of 70% and 30%, respectively in comparison to 89% and 60%, respectively in ICE alone at 24 months. Grade≥3 adverse effects were also higher i.e., 37% in the BICE group in comparison to 28% in ICE alone. Table.1
Conclusion:
Concurrent treatment with ICE and ICIs was well tolerated and was associated with improved effectiveness. BV-ICE achieved a greater CR regardless of frontline treatment and toxicity was manageable. PAN-ICE combination also produced high CR rate but at the cost of greater bone marrow toxicity. Investigation of PAN with less myelosuppressive agent can be considered for future trials. Due to the lack of significant improvement in outcomes of BICE as compared to ICE, this trial was stopped. More randomized double-blind large-scale phase III studies for further evaluation are needed.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal